Dengue fever is caused by four distinct serotypes of the dengue virus: DENV-1, DENV-2, DENV-3, and DENV-4. It is the most widespread mosquito-borne viral disease globally. Due to the risk of antibody-dependent enhancement (ADE), the development of a tetravalent vaccine capable of simultaneously covering all four serotypes has become essential.
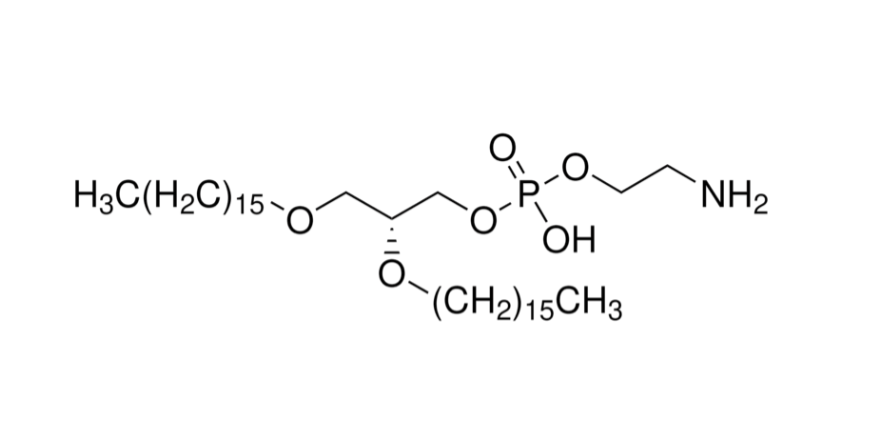
The mRNA vaccine platform offers rapid design, strong immunogenicity, and flexible multivalent combinations. Its success, however, relies on an efficient delivery system, with lipid nanoparticles (LNPs) being the gold standard. In particular, SM-102, a clinically validated ionizable lipid used in COVID-19 vaccines, has demonstrated excellent safety and delivery efficiency, making it a leading candidate for tetravalent dengue mRNA vaccine construction.
I. Advantages of SM-102 in Tetravalent Dengue mRNA Vaccine Delivery
1. Efficient Delivery and Balanced Antigen Expression
- Protonates under acidic conditions to effectively encapsulate mRNA molecules.
- Returns to near-neutral charge at physiological pH, reducing nonspecific protein interactions.
- Ensures balanced delivery of mRNAs from all four dengue serotypes, preventing immune imbalance caused by disproportionate antigen expression.
2. Favorable Tissue Distribution and Immune Activation
- Rapidly distributes to lymph nodes post-injection, enhancing dendritic cell uptake and antigen presentation.
- Persists in muscle and lymphatic tissue, enabling sustained immune stimulation and broader tetravalent protection.
3. Predictable Metabolism and Low Toxicity
- Degraded by esterases into fatty acids and neutral metabolites with well-characterized metabolic pathways.
- Minimizes accumulation in liver and spleen, lowering long-term toxicity risks.
- Meets FDA safety requirements for biodegradable ionizable lipids.
4. Flexible Formulation Optimization
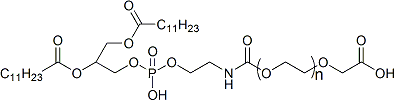
- By adjusting ratios of SM-102, DSPC, cholesterol, and PEG-lipids, particle size, surface charge, and release rate can be finely tuned.
- Provides formulation plasticity critical for balancing antigen expression across multivalent mRNA vaccines.
II. Workflow for Constructing Tetravalent Dengue mRNA Vaccine with SM-102
1. Antigen Design and mRNA Synthesis
- Antigen selection: Dengue virus E (Envelope) and prM proteins as primary immunogens; optional inclusion of NS1 fragments to enhance cellular immunity.
- mRNA optimization:
- Optimized 5′/3′UTRs for stability and translation efficiency.
- Incorporation of nucleoside modifications (e.g., N1-methyl-pseudouridine) to reduce innate immune activation.
- Tailored Cap structures and Poly(A) lengths to maximize protein translation.
2. SM-102 LNP Formulation Development
- Core composition: SM-102 + DSPC + cholesterol + PEG-lipid.
- Formulation targets:
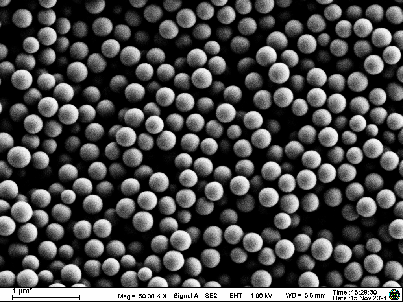
- Particle size: 80–100 nm for efficient lymph node uptake.
- Encapsulation efficiency: >90%.
- Zeta potential: Neutral to slightly negative, minimizing toxicity.
- Balanced molar ratios of all four mRNAs to avoid immune response bias.
3. In Vitro and In Vivo Evaluation
- In vitro: Translation efficiency and antigen protein expression.
- Small animals: Immunogenicity evaluation and ADE risk monitoring.
- Non-human primates: Long-term protection and immune balance validation.
4. Process Scale-Up and Stability Studies
- Scale-up: Microfluidic mixing technology for reproducible batch manufacturing.
- Stability: Freeze-drying or ultra-low temperature storage to maintain LNP integrity.
- Quality control: Size distribution, encapsulation efficiency, mRNA integrity, and purity assessment.
III. Taskcm’s SM-102-Based Service Capabilities
As a specialized platform for nucleic acid drug delivery and process development, Zhongxin Kangming offers comprehensive services to accelerate tetravalent dengue mRNA vaccine development:
1. LNP Formulation Development & Optimization
- SM-102-based LNP screening and high-throughput optimization.
- Balancing encapsulation and release across four mRNAs.
- DoE-driven formulation design strategies.
2. In Vitro and In Vivo Delivery Evaluation
- mRNA expression profiling and antigen detection.
- Small animal immunogenicity evaluation and ADE risk monitoring.
- Biodistribution and pharmacokinetics studies.
3. Process Scale-Up & Quality Control
- Microfluidic LNP preparation and pilot-scale manufacturing.
- GLP/GMP-compliant quality control: particle size, encapsulation, purity, stability.
- Screening cryoprotectants for long-term stability assurance.
4. Preclinical & Regulatory Support
- PK/PD and safety studies compliant with regulatory guidelines.
- PBPK modeling to predict clinical outcomes.
- IND submission support including CMC and preclinical data packages.
IV. Conclusion
SM-102 provides high-efficiency delivery, balanced pharmacokinetics, and excellent safety, positioning it as a critical enabler for tetravalent dengue mRNA vaccine development.
With a robust delivery technology platform, GMP manufacturing systems, and comprehensive preclinical evaluation expertise, Taskcm offers end-to-end services—from molecular design to regulatory submission—to accelerate clinical translation and market entry of tetravalent dengue mRNA vaccines.