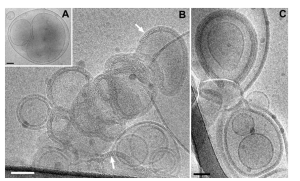
In recent years, mRNA technology has been increasingly applied in vaccine and drug development. Following the COVID-19 pandemic, the potential of mRNA platforms has gradually expanded to major infectious diseases such as seasonal influenza, RSV, and CMV. Among these, seasonal influenza vaccines remain one of the most valuable and in-demand directions. Due to the rapid mutation of influenza viruses and the long production cycle of traditional vaccines, there is an urgent need for more flexible and efficient vaccine production methods. mRNA-based influenza vaccine candidates have attracted attention because of their short development cycle, tunable immunogenicity, and standardized production processes.
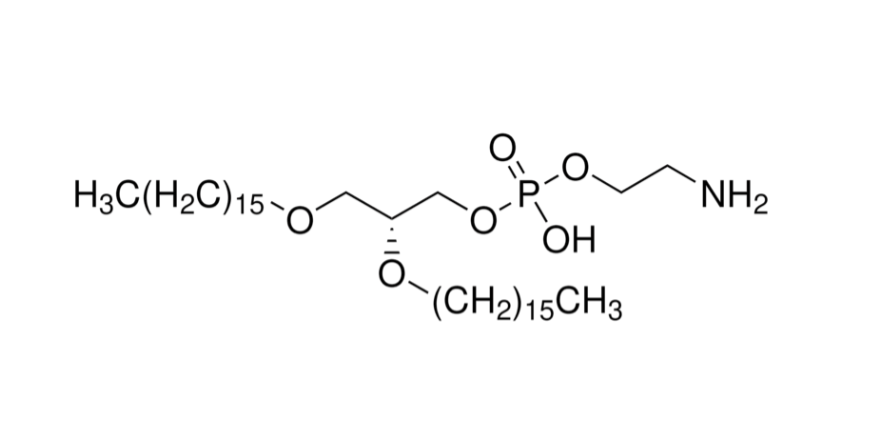
In this context, lipid nanoparticles (LNPs) are a critical delivery tool for mRNA vaccines. They not only protect mRNA molecules from degradation in vivo but also deliver them precisely to host cells, enabling antigen protein expression and eliciting immune responses. As a core ionizable lipid in LNPs, SM-102 has demonstrated unique advantages in the development of seasonal influenza vaccines.
Taskcm showcases its highlights in kilogram-scale SM-102 supply, raw material security, and customized service capabilities, providing practical solutions for vaccine R&D institutions and pharmaceutical companies.
I. Unique Advantages of SM-102 in Seasonal Influenza mRNA Vaccines
1. Efficient mRNA Encapsulation and Delivery
SM-102 is an ionizable lipid with a precisely tuned pKa, allowing it to carry a positive charge under acidic conditions and form stable complexes with negatively charged mRNA molecules. During formulation, SM-102 self-assembles with other helper lipids (cholesterol, DSPC, PEG-lipid) into nanoparticles, effectively encapsulating mRNA.
This structure not only increases mRNA encapsulation efficiency but also protects it from nuclease degradation in vivo, significantly enhancing delivery efficiency.
2. Optimized Cellular Uptake and Endosomal Escape
SM-102 LNPs can enter cells via endocytosis. Its ionizable property enables rapid charge acquisition in the acidic environment of endosomes, disrupting the endosomal membrane and releasing mRNA into the cytoplasm. This process enhances translation efficiency and increases antigen protein expression. For seasonal influenza vaccines, this means stronger immunogenicity with lower dosage requirements.
3. Improved Immunogenicity and Tolerability
The key goal of influenza vaccines is to induce broad and durable immune responses. Studies show that SM-102-based LNPs can effectively activate dendritic cells and macrophages, enhance antigen presentation, and induce stronger neutralizing antibody and T-cell responses. Compared with traditional adjuvants, SM-102 demonstrates higher safety and controllable local inflammation, making it suitable for clinical applications.
4. Support for Multivalent Antigen Delivery
Seasonal influenza vaccines are often multivalent, covering A/H1N1, A/H3N2, B/Yamagata, B/Victoria strains, etc. SM-102’s excellent complexation properties allow it to stably deliver multiple mRNA molecules simultaneously, laying a solid foundation for quadrivalent or next-generation broad-spectrum influenza vaccines.
II. Why Choose SM-102 over Other Lipids?
Compared to lipids like ALC-0315 or MC3, SM-102 offers several advantages:
- Extensive clinical validation: SM-102 has entered clinical trials in multiple mRNA vaccines (including influenza candidate mRNA-1010), with reliable safety and efficacy data.
- Mature and stable manufacturing: Its synthesis route and quality standards have been industrially validated, facilitating large-scale production.
- Broad applicability: It is suitable not only for influenza vaccines but also for RSV, CMV, and emerging infectious disease vaccines.
These features make SM-102 one of the most representative core lipids in mRNA vaccine platforms, especially suitable for rapid iteration and large-scale supply of influenza vaccines.
III. Taskcm’s Advantages in SM-102 Supply and Services
1. Kilogram-scale High-quality SM-102 Production
- Scaled-up synthesis: A mature kilogram-scale synthesis and purification route ensures stable processes, high yields, and batch-to-batch consistency.
- High purity control: Multi-step purification ensures lipid purity >99%, effectively removing residual solvents and by-products.
- Quality compliance: Products can be manufactured according to GMP standards, with full analytical reports including HPLC, NMR, MS, and residual solvent testing.
- Flexible supply: From gram-scale R&D samples to kilogram-scale pilot materials, meeting diverse customer needs from lab validation to clinical production.
2. Raw Material Security and Global Supply Chain
- Long-term stable supply: Independent production facilities and raw material reserves ensure uninterrupted supply for vaccine developers.
- International registration support: DMF and complete registration documentation are available to support submissions in China, the U.S., EU, and other markets.
- Rapid delivery: Shortens R&D timelines, helping partners seize clinical and market opportunities.
3. Customized Formulation and Process Development
- Formulation optimization: Tailored SM-102/mRNA ratios, particle size, and charge to ensure delivery efficiency and stability based on antigen properties.
- Pilot scale-up: Advanced micro-mixing and continuous flow equipment ensure consistency from small-scale to kilogram-scale production.
- Stability studies: Provides systematic accelerated and long-term stability evaluation, with optimized storage and transportation conditions.
- Collaborative development: Works with clients’ R&D teams or CROs to support in vivo experiments and immunological assessments.
4. One-stop Solution
Taskcm provides a full-chain service of “raw material + process development + pilot scale-up + regulatory support”, helping clients transition rapidly from laboratory research to clinical applications.
IV. Conclusion
The seasonal influenza vaccine market is vast, and mRNA technology offers new development opportunities. As a critical lipid supporting mRNA platforms, SM-102 demonstrates unparalleled advantages in delivery efficiency, safety, and clinical validation.
With kilogram-scale high-quality SM-102 supply, a complete quality system, and flexible customized process development services, Taskcm is able to provide full-chain support to global vaccine developers. We look forward to collaborating with partners to advance the R&D and industrialization of seasonal influenza mRNA vaccines, contributing to global public health.