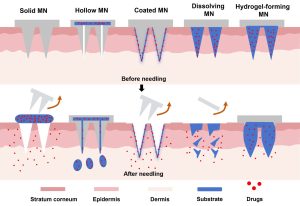
With the continuous advancement of vaccine technologies, the traditional injection approach is increasingly revealing its limitations in terms of safety, compliance, and distribution efficiency. Needle phobia, strained medical resources, and a strong reliance on cold-chain logistics all hinder vaccine accessibility on a global scale. As a novel, painless, and user-friendly delivery strategy, microneedle patches are emerging as a breakthrough in vaccine administration.
PVA/Chitosan (Polyvinyl Alcohol/Chitosan) composite microneedles, as representatives of biodegradable materials, not only offer excellent mechanical strength and biocompatibility but also enable stable encapsulation and sustained release of vaccines. This provides a practical solution to enhance vaccine absorption efficiency while simplifying vaccination procedures.
Product Name
PVA/Chitosan Biodegradable Vaccine Delivery Microneedle System
Product Overview
This product is a minimally invasive transdermal vaccine delivery patch composed of a microneedle array built from polyvinyl alcohol and chitosan. The needle matrix encapsulates stabilized vaccine antigens and is suitable for localized administration of vaccines such as influenza, HPV, rabies, and hepatitis B.
In this field, Taskcm provides customized microneedle design services for different vaccine types, including microneedle array dimensions, needle length, density, and material composition ratios, to meet diverse drug delivery needs. At the same time, we have robust mold fabrication and casting capabilities to support batch production and timely delivery.
Technical Specifications
- Microneedle length: 300–800 μm (customizable)
- Needle tip diameter: ≤30 μm
- Vaccine loading capacity: customizable, up to several hundred micrograms
- Dissolution time: completely dissolves within 2–10 minutes
- Biodegradation time: metabolized and cleared within 12–24 hours in vivo
Material Composition
- Matrix materials: Polyvinyl Alcohol (PVA), Chitosan
- Stabilizers: trehalose, glycerol, or other excipients depending on vaccine type
- Optional excipients: Polyvinylpyrrolidone (PVP), gelatin, etc.
Physical Properties
- Sufficient strength to penetrate the epidermis
- Good flexibility and skin conformability
- Rapid dissolution with minimal residue risk
- User-friendly design, portable, and convenient to use
Drug Loading and Stability Strategies
Depending on the stability and release requirements of different vaccines, we offer multiple encapsulation strategies, including co-loading of hydrophilic and lipophilic agents, microenvironmental stabilizer adjustment, and protective excipient formulations to ensure vaccine activity is preserved during microneedle fabrication and storage.
Product Advantages
Performance Advantages
- Minimally invasive and painless, reduces injection-related anxiety
- Controlled release for improved vaccine utilization
- Potential for room-temperature storage, easing cold-chain reliance
- Self-dissolving, no needle removal or medical waste management required
Core Functionalities
- Precise intradermal delivery, activating abundant antigen-presenting cells
- Supports stable vaccine encapsulation, sustained release, and targeted delivery
- Biodegradable, with no residual processing needed
Competitive Comparison
- Compared with traditional syringes: easier to operate, safer, and more compliant
- Compared with polylactic acid or metal microneedles: lower cost, no recycling issues
- Supported by Taskcm’s robust scale-up platform, enabling seamless transition from small-batch lab production to industrial-scale manufacturing
Economic Advantages
- Reduces healthcare labor costs
- Enables self-administration and remote distribution models
- Minimizes cold-chain transportation needs, significantly lowering logistics expenses
Cost Efficiency
- Mass production readiness ensures unit cost is significantly lower than conventional injection consumables
- Designed for single-use with compatibility for automated manufacturing and packaging
Investment Value
- Ideal for vaccine enterprises to expand into innovative dosage forms
- Serves as a versatile platform for multivalent vaccines or combination therapies, extending product lifecycle
- Aligned with global vaccination trends of digitization, portability, and non-invasive delivery
Custom Services
We also offer antibody-conjugated microneedle customization, suitable for multivalent vaccines or targeted immuno-enhancement platforms. By covalently coupling antigens or adjuvants to microneedles, we further enhance immune recognition efficiency.
Implementation Roadmap
Design Phase:
- Tailor microneedle structure and density based on vaccine type and dosage
- Adjust PVA-to-chitosan ratios to optimize dissolution and antigen stability
- Provide antigen-conjugation and antibody-adaptation strategies for personalized development
Taskcm offers end-to-end microneedle development services, including antigen compatibility design, drug encapsulation strategies, controlled-release layer engineering, and bioactivity validation—ensuring translational readiness from the outset.
Manufacturing Phase:
- Mold casting and automated filling ensure product consistency
- Pilot-scale platform and GMP-grade production lines support projects at various stages
- Capabilities for sample generation, validation batch production, and tech transfer
Conclusion
PVA/Chitosan microneedle technology is emerging as a transformative innovation in vaccine delivery. With its safe, user-friendly, and biodegradable properties, it has the potential to disrupt conventional injection-based vaccination, boost vaccine accessibility, and promote more patient-centered public health solutions.
Taskcm is dedicated to providing comprehensive microneedle solutions for vaccine and biologics developers, covering every stage from product design, antigen encapsulation, and conjugation strategies to pilot-scale expansion and GMP production. We welcome collaborations with research institutions, vaccine companies, and healthcare organizations to jointly bring this cutting-edge technology from lab to clinic and market.