Advantages and Applications of DLin-MC3-DMA as a Pharmaceutical Excipient: Taskcm Offers Kilogram-Scale Production Solutions
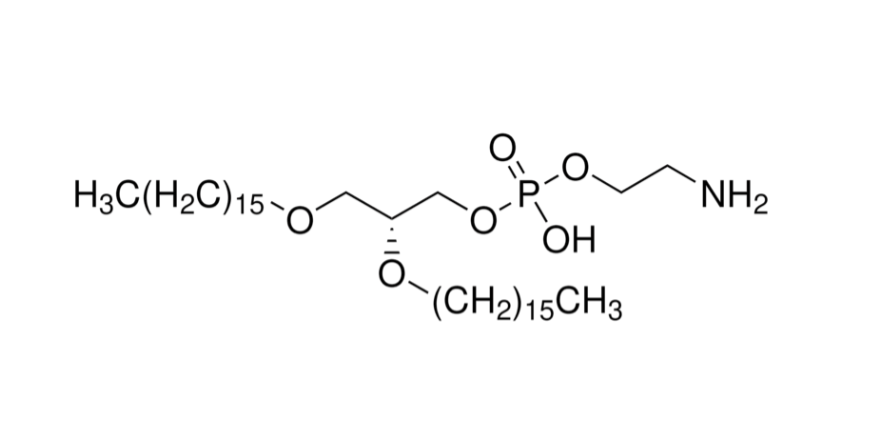
With the rapid development of nucleic acid therapeutics and mRNA vaccines, lipid nanoparticles (LNPs) have become the most essential delivery platform for in vivo applications. Among them, DLin-MC3-DMA (commonly referred to as MC3) is the most representative ionizable cationic lipid and is widely recognized as the “gold standard” carrier. As the key excipient successfully translated into the marketed nucleic acid drug Onpattro® (patisiran), MC3 established the foundation of ionizable lipids in drug delivery.
Leveraging advanced synthesis technology and stringent quality management systems, Taskcm is capable of supplying DLin-MC3-DMA in kilogram-scale packaging. From laboratory research to clinical translation and industrial supply, we support partners in achieving breakthroughs in nucleic acid therapeutics and vaccine development.
I. Core Advantages of DLin-MC3-DMA
Optimal pKa for precise endosomal release
The apparent pKa of DLin-MC3-DMA is approximately 6.2–6.5, falling exactly within the optimal “window” for efficient delivery. Under physiological pH in blood circulation, MC3 remains nearly neutral, avoiding nonspecific toxicity and immune activation. Once inside acidic endosomes, MC3 becomes protonated and positively charged, promoting membrane fusion and rupture, thereby enabling highly efficient nucleic acid release. This feature underpins the molecular basis of MC3’s superior delivery performance.
Extremely low effective dose
Preclinical studies have shown that MC3 can achieve potent gene silencing at ultra-low doses in both rodents and non-human primates. For example, in a coagulation factor VII liver model, the ED50 was as low as 0.005 mg/kg; in a transthyretin (TTR) model in non-human primates, the ED50 was around 0.03 mg/kg. This highlights MC3’s ability to enhance therapeutic efficiency while minimizing potential toxicity risks.
Clinically validated reliability
MC3 is the critical excipient in Onpattro®. As the world’s first approved siRNA-LNP drug, Onpattro’s success not only represents a milestone in RNAi research but also provides strong proof of MC3’s clinical feasibility and safety. Both the FDA and EMA review documents explicitly list MC3 as a novel excipient, with detailed requirements for its pharmacokinetics, safety, and quality control, providing valuable reference for subsequent drug development.
Mature processes and regulatory documentation
Due to its extensive study and application, MC3 benefits from well-established synthetic routes, quality control criteria, pharmacokinetic data, and analytical methods available in literature and regulatory filings. This reduces uncertainty for pharmaceutical companies during clinical registration and offers a robust technical reference for new drug development.
II. Research Journey: From Early Screening to Clinical Translation
Early screening (academic stage)
In 2012, Jayaraman et al. systematically studied the correlation between LNP delivery efficiency and the pKa of ionizable lipids. Through large-scale synthesis and testing, MC3 was identified, showing markedly superior efficiency compared to earlier lipids such as DLin-KC2-DMA. This foundational study positioned MC3 as a prime clinical candidate.
Preclinical validation (animal studies)
MC3-LNPs demonstrated strong gene silencing efficacy and favorable safety in rodent and non-human primate models. In liver-targeting experiments, MC3-LNPs achieved >90% knockdown of target genes, while maintaining low dose and low toxicity profiles.
Clinical application (the birth of Onpattro®)
Alnylam successfully developed patisiran into a commercial drug using MC3-LNPs, which was approved by both FDA and EMA in 2018. Regulatory reviews documented detailed data on MC3’s manufacturing process, pharmacokinetics, residual analysis, and stability, further solidifying MC3’s industrial significance.
Further development (comparisons and optimization)
Although newer ionizable lipids such as ALC-0315 and SM-102 have been widely used in mRNA vaccines, MC3 continues to serve as the benchmark lipid in comparative studies. Researchers investigate differences in immunogenicity, biodistribution, and biodegradation, providing insights for formulation optimization.
III. Industrial Scale-Up and Quality Control Considerations
Stable and reproducible formulation composition
The standard Onpattro formulation employs MC3:DSPC:Cholesterol:PEG-lipid = 50:10:38.5:1.5 (mol%). This ratio has become a widely referenced framework for LNP formulations, offering excellent reproducibility and scalability.
Clear quality control standards
MC3 must meet high-purity standards (≥98%), low residual solvents, controlled isomer ratios, and stringent stability requirements. Comprehensive analytical methods (HPLC, LC-MS, NMR, etc.) are essential to ensure batch-to-batch consistency.
Key scale-up parameters
From gram-scale to kilogram-scale production, critical steps include microfluidic mixing parameters, solvent removal, sterile filtration, and cryopreservation. Any deviation may directly affect particle size distribution, encapsulation efficiency, and stability.
IV. Taskcm’s Kilogram-Scale Production and Service Advantages
As a leading domestic supplier of lipid raw materials and delivery platforms, Taskcm is committed to providing global customers with end-to-end support from R&D to industrialization.
- Kilogram-scale supply capability
We stably supply kilogram-scale batches of DLin-MC3-DMA, meeting the demands of large-animal studies, toxicology testing, and Phase I/II clinical projects.
- Stringent quality management
Our company operates under internationally aligned quality systems, providing comprehensive testing reports including purity, content, moisture, residual solvents, heavy metals, and stability. Customized COAs and regulatory registration documents are available upon request.
- Process and technical support
In addition to raw material supply, we provide solubility recommendations, storage and transport solutions, microfluidic process parameter references, and critical quality attribute (CQA) identification, helping partners shorten timelines from R&D to regulatory filing.
- Customized services
We offer process development, scale-up trials, and CDMO integration support to help clients address potential challenges in LNP formulation and industrialization.
V. Application Prospects and Partnership Value
- siRNA therapeutics: MC3-LNPs remain one of the most efficient carriers for liver-targeted delivery, particularly suitable for rare diseases or hepatic disorders.
- mRNA vaccines and therapeutics: Despite the emergence of new ionizable lipids, MC3 remains the benchmark lipid for comparative studies, formulation development, and methodological optimization.
- Preclinical and toxicology studies: Kilogram-scale supply ensures the smooth execution of large-animal studies and toxicological evaluations, reducing risks associated with raw material shortages.
Conclusion
DLin-MC3-DMA, with its unique physicochemical properties, high delivery efficiency, and successful application in Onpattro®, has become a classical representative of ionizable lipids. From early academic discovery to clinical translation, MC3 has been comprehensively validated.
As the demand for nucleic acid therapeutics and mRNA vaccines continues to grow, reliable raw material supply is critical for success. With kilogram-scale production capabilities, rigorous quality management, and robust technical support, Taskcm is committed to collaborating with global partners to accelerate innovation and drive nucleic acid drug and vaccine development to new heights.