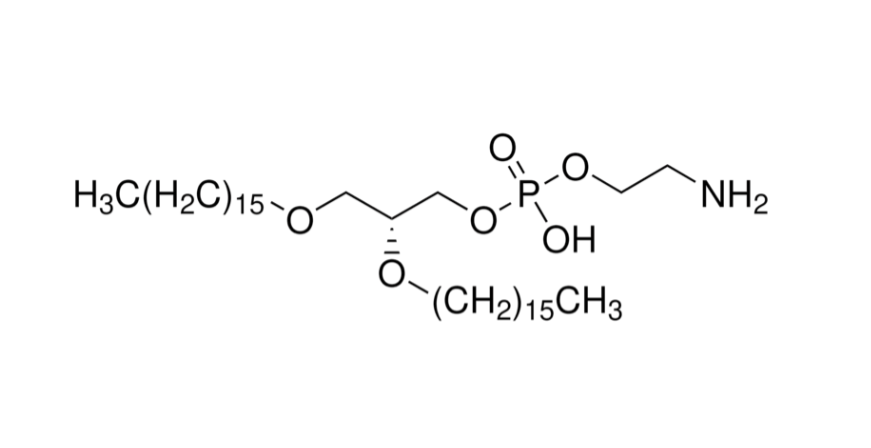
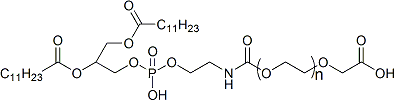
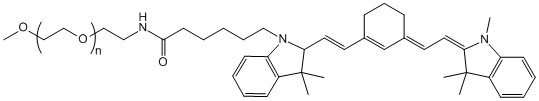
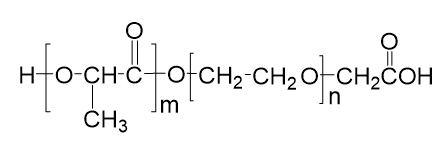
With the growing demand for safer and more effective gout treatments, Taskcm has successfully developed colchicine-loaded silk fibroin microneedles, offering a novel transdermal delivery approach for acute gouty arthritis. This breakthrough not only addresses the narrow therapeutic window and gastrointestinal side effects of traditional oral colchicine formulations but also marks a significant step toward the industrialization and commercialization of microneedle-based drug delivery systems.
1. Silk Fibroin Microneedle Technology: Biocompatible, Dissolvable, and Effective
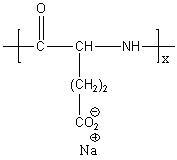
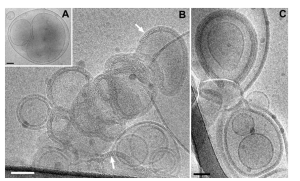
The colchicine silk fibroin microneedle system leverages the natural biocompatibility, mechanical strength, and degradability of silk fibroin to achieve precise and sustained drug delivery through the skin.
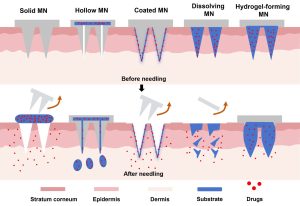
Two microneedle architectures have been developed:
- Integrated Microneedles: Both needle tips and baseplates are made of silk fibroin, enabling full biodegradability.
- Separated Microneedles: The needle tips are composed of silk fibroin while the backing layer is made of PVP K90, allowing for rapid release after skin insertion.
In in vitro permeation studies, the separated microneedle formulation exhibited a higher cumulative permeation rate and sustained release even after backing removal.
In in vivo efficacy tests using a rat model of acute gouty arthritis, transdermal administration of colchicine microneedles significantly reduced ankle swelling and decreased serum inflammatory cytokines IL-1β and TNF-α, outperforming both the model and oral administration groups (P < 0.01).
These findings confirm that colchicine silk fibroin microneedles provide a potent anti-inflammatory effect via the skin, offering a safer and more efficient route of administration for gout therapy.
2. Kilo-Scale Manufacturing Capability: Bridging Laboratory Innovation to Industry
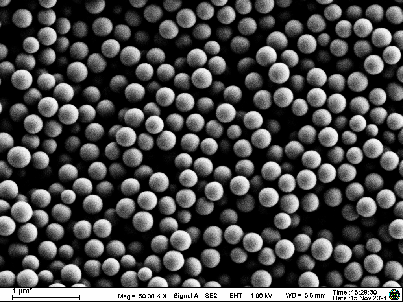
Taskcm has established a dedicated microneedle manufacturing and process optimization platform, achieving kilo-scale preparation capacity for silk fibroin microneedles.
Through advanced molding, casting, and drying techniques, the company ensures:
- Consistent microneedle geometry and mechanical strength;
- Controlled drug loading efficiency and uniformity;
- Scalable and reproducible batch production under GMP-compliant environments.
Raw silk fibroin materials meet ISO 10993 biocompatibility standards, and full analytical documentation (COA, residual solvent analysis, morphology, and thermal stability reports) is provided. This ensures traceability and regulatory readiness for clients entering preclinical and IND application stages.
3. End-to-End CDMO Service for Microneedle System Development
As a leading developer and manufacturer of lipid and polysaccharide-based drug delivery systems, Taskcm offers a comprehensive CDMO (Contract Development and Manufacturing Organization) service for microneedle formulations—covering formulation design, process development, scale-up, quality control, and regulatory support.
Formulation & Process Development
- Optimization of microneedle mechanical strength, dissolution rate, and drug loading.
- Evaluation of molding, centrifugal casting, and 3D printing-based fabrication methods.
- Development of controlled-release and responsive-release systems through protein crosslinking or polymer blending.
Quality Analysis & Characterization
- Utilization of SEM, FTIR, XRD, DSC, TGA, HPLC, and GPC to assess microneedle morphology, structural integrity, and stability.
- Comprehensive analysis of drug encapsulation efficiency, release kinetics, and biodegradation behavior.
Pilot Scale & Technology Transfer
- Equipped with GMP-compliant pilot workshops with controlled environments for consistent batch validation.
- Support for CPP (Critical Process Parameter) research and process reproducibility studies.
- Technology transfer assistance and equipment selection consulting for industrial production.
Regulatory & CMC Support
- Preparation of CMC documentation, quality study reports, and submission-ready technical files.
- Expertise in both drug registration and medical device filing pathways, ensuring compliance and accelerating regulatory approval.
4. Clinical and Commercial Potential: Toward the Future of Gout Treatment
The colchicine silk fibroin microneedle system demonstrates high potential for treating acute gouty arthritis, offering localized, sustained drug delivery without the gastrointestinal irritation associated with oral administration.
Beyond gout therapy, Taskcm is expanding silk fibroin microneedle applications into dermatology, vaccine delivery, pain management, and oncology, reinforcing its role as a pioneer in biopolymer-based transdermal technologies.
5. Conclusion: From Innovation to Industrialization
Taskcm stands at the forefront of microneedle system industrialization in China, capable of delivering kilo-scale manufacturing, full-spectrum CDMO services, and regulatory guidance for silk fibroin microneedle formulations.
By combining material innovation, process engineering, and commercial scalability, Taskcm provides a one-stop solution—from silk fibroin raw material → microneedle formulation → pilot scale-up → IND registration—helping global partners accelerate the translation of laboratory discoveries into clinical and commercial success.