With the rapid rise of nucleic acid therapeutics and mRNA vaccines, lipid nanoparticles (LNPs) have emerged as the most critical in vivo delivery platform. Among them, SM-102, ALC-0315, and MC3 are the most widely used ionizable lipids, each with unique advantages in pharmacokinetics, safety, delivery efficiency, and therapeutic applications.
At Taskcm, we help research teams select the optimal lipid delivery platform—tailored to drug modality (mRNA vs. siRNA), disease indication (acute infection vs. chronic disease), and development goal (immune activation vs. long-term safety)—to accelerate R&D and reduce risks across the entire pipeline.
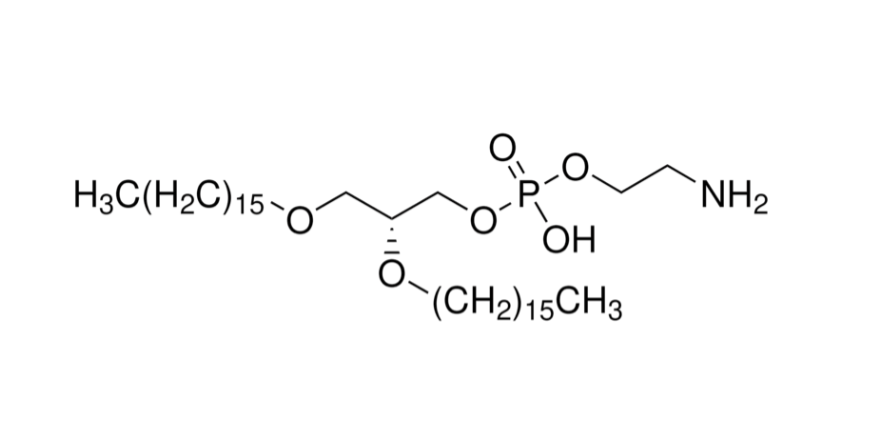
1. SM-102 — The Balanced Lipid for Safe and Stable mRNA Delivery
Advantages:
- Balanced pharmacokinetics: Neutral at physiological pH, reducing nonspecific binding and clearance, extending circulation time.
- Favorable biodistribution: Lower hepatic accumulation, sustained presence in muscle and lymph nodes—ideal for vaccines and local therapies.
- Predictable metabolism: Ester bonds hydrolyzed by esterases, ensuring clear metabolic pathways and low toxicity.
- Formulation flexibility: Adjustable ratios in LNPs allow fine-tuning of half-life and biodistribution.
Best suited for: mRNA vaccines and protein replacement therapies, where both expression efficiency and safety are required.
Taskcm Services – SM-102 Platform
- Custom SM-102 LNP formulation development
- Preclinical PK/PD studies
- GLP-compliant toxicology and safety assessments
- IND filing support
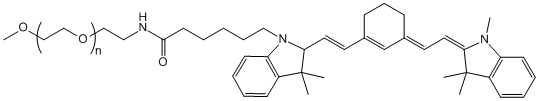
2. ALC-0315 — The High-Efficiency Lipid for Vaccines and Immunotherapy
Advantages:
- High delivery efficiency: Optimized structure enhances cellular uptake and mRNA expression.
- Rapid immune activation: Demonstrates strong immunogenicity in preclinical models, inducing robust immune responses.
- Clinically validated: The success of Pfizer/BioNTech’s COVID-19 vaccine provides extensive safety and efficacy data in animals and humans.
Limitations:
- Higher potential for inflammatory responses compared to SM-102.
- More complex synthesis and relatively challenging scale-up.
Best suited for: Infectious disease vaccines and cancer immunotherapy, where fast and strong immune responses are required.
Taskcm Services – ALC-0315 Platform
- High-efficiency ALC-0315 LNP formulation screening
- Immunogenicity testing in animal models
- Toxicology and inflammation profiling
- End-to-end CRO support for vaccine development
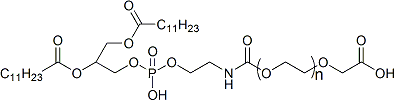
3. MC3 — The Safety Standard for siRNA and Gene Silencing Therapies
Advantages:
- Established safety: FDA-approved in Onpattro (the first siRNA drug), with the most comprehensive safety and metabolic dataset.
- Low toxicity: Ester bond hydrolysis produces safe metabolites; robust preclinical toxicology data.
- Mature processes: Well-established analytical and manufacturing workflows, enabling efficient scale-up.
Limitations:
- Less efficient for mRNA delivery compared with SM-102 and ALC-0315.
- Limited application in vaccines and protein replacement therapies.
Best suited for: siRNA-based therapies and RNAi drugs targeting chronic diseases requiring long-term safe delivery.
Taskcm Services – MC3 Platform
- Custom MC3 siRNA LNP formulation and optimization
- GLP toxicology and long-term safety assessments
- PK/PD and biodistribution modeling
- CMC and industrial-scale manufacturing support
Conclusion
- SM-102: Balanced lipid—optimal for mRNA vaccines and protein replacement therapies.
- ALC-0315: High-efficiency lipid—ideal for vaccines and immunotherapies requiring rapid immune activation.
- MC3: Safe lipid—the gold standard for siRNA and gene silencing drugs with long-term treatment needs.
By leveraging these three proven lipid delivery platforms, Taskcm provides tailored end-to-end solutions that accelerate the transition from discovery → preclinical → clinical development, helping clients reduce risk, improve efficiency, and achieve successful translation of nucleic acid medicines.