HSA-PLGA Nanoparticles: Prescription Development and Optimization in CDMO Services
In the field of pharmaceutical R&D, HSA-PLGA (human serum albumin–poly(lactic-co-glycolic acid)) nanoparticles have emerged as a novel platform for drug delivery and controlled release, drawing increasing attention. Their unique biocompatibility, biodegradability, and compatibility with various drugs endow them with broad application prospects in advanced drug delivery systems.
I. Advantages in Stability and Biocompatibility
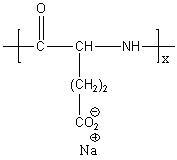
HSA, as a natural protein, possesses excellent biocompatibility and low immunogenicity. When combined with PLGA, the resulting HSA-PLGA nanoparticles retain the intrinsic benefits of HSA while gaining enhanced stability and biodegradability from PLGA. This design allows the nanoparticles to degrade slowly in vivo, enabling sustained drug release, reducing adverse side effects, and improving therapeutic efficacy.
In this regard, Taskcm offers expertise in the design and customization of albumin-based nanoparticles with tailored particle sizes and structures, supporting drug modification and encapsulation studies to improve drug stability and therapeutic performance in vivo.
1. Initial Prescription Design
- Polymer selection: Choose optimal PLGA molecular weight and lactide/glycolide ratio based on drug physicochemical properties (hydrophobicity, molecular weight, stability).
- HSA coating strategy: Apply physical adsorption, chemical conjugation, or self-assembly methods to enhance nanoparticle stability and targeting ability.
- Drug-specific formulation: Develop tailored encapsulation and release strategies for small-molecule drugs, protein therapeutics, and nucleic acids.
2. Critical Process Parameter Optimization
- Emulsification/nanoprecipitation optimization: Control particle size distribution and PDI through adjustment of solvent type, stirring rate, and surfactant concentration.
- Encapsulation efficiency: Improve drug stability and loading capacity by optimizing oil-to-water phase ratios and drug pretreatment conditions.
- Release profile control: Fine-tune PLGA degradation rate and HSA coating thickness to achieve personalized fast-release or sustained-release profiles.
3. Surface Functionalization Strategies
- HSA conjugation: Extend circulation half-life and enhance tumor targeting through HSA coupling.
- Ligand modification: Incorporate antibodies, peptides, or polysaccharides for precision drug delivery.
- Stability enhancement: Reduce opsonization and improve immune evasion via HSA surface shielding.
4. Prescription Screening and Evaluation
- Physicochemical characterization: Particle size, PDI, and zeta potential measurements.
- In vitro release assays: Comparative evaluation of release profiles across formulations.
- Stability testing: Assess effects of temperature, pH, and freeze-drying/rehydration on nanoparticle integrity.
- Biocompatibility validation: Cytotoxicity and hemocompatibility testing.
5. Scalability and Process Transfer
- Ensure formulation scalability across laboratory, pilot, and GMP production levels.
- Provide process transfer and feasibility assessment reports to accelerate preclinical development timelines.
II. Clinical Prospects and Applications
Although still in the research phase, HSA-PLGA nanoparticles demonstrate significant advantages in enhancing drug stability, controlling release kinetics, and enabling targeted delivery. These benefits point to promising clinical applications, particularly in anticancer therapy, vaccine delivery, and protein therapeutics.
Taskcm continues to invest in this direction, offering integrated CDMO services from early-stage R&D to large-scale production, bridging the gap between laboratory innovation and real-world clinical application.
In summary, HSA-PLGA nanoparticles, as a novel drug delivery and controlled release platform, are becoming a research hotspot in pharmaceutical development. With continuous innovation and industrial support, they are poised to play an important role in clinical practice, offering patients safer and more effective treatment options.
III. Application Case Study
Title: Construction of ASODN-Protamine-HSA-PLGA Nanoparticles and Preliminary Study of Their In Vitro Nuclear Targeting
Abstract:
Objective: To investigate the preparation of a novel non-viral vector, ASODN-Protamine-HSA-PLGA nanoparticles, and their potential for nuclear targeting in vitro.
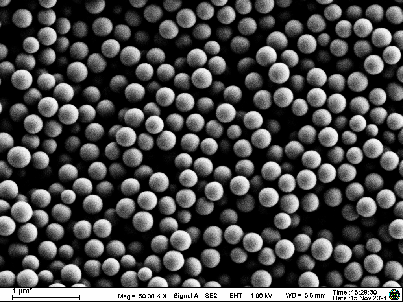
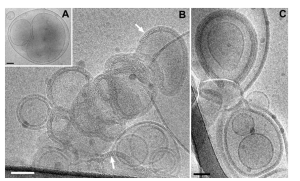
Methods: Antisense oligodeoxynucleotides (ASODNs) were condensed with protamine sulfate and human serum albumin (HSA), followed by encapsulation within PLGA using a double-emulsion solvent evaporation method to prepare ASODN-P/H-PLGA nanoparticles. Transmission electron microscopy (TEM) was employed to observe morphology; particle size, polydispersity index (PDI), and zeta potential were measured with a laser particle analyzer; encapsulation efficiency was determined by a two-step method; cytotoxicity was assessed via MTT assay; and cellular uptake and nuclear targeting were analyzed by confocal laser scanning microscopy in Hep-2 laryngeal squamous carcinoma cells.
Results: The prepared nanoparticles displayed uniform, spherical morphology with an average size of 128 nm, PDI of 0.234, and average zeta potential of –23.3 mV. The average encapsulation efficiency was 78.45%.