SM-102: The Core Lipid for PK Optimization in Nucleic Acid Drugs
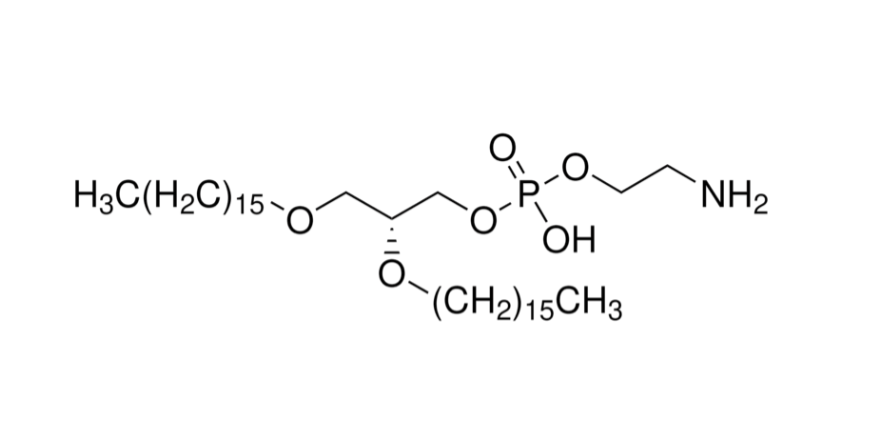
With the rapid rise of mRNA vaccines and nucleic acid therapeutics, lipid nanoparticles (LNPs) have become the most important delivery platform. Among them, SM-102 is a representative ionizable lipid, widely validated in Moderna’s COVID-19 vaccine and other global applications. Pharmacokinetics (PK) is a critical step in evaluating LNP/mRNA systems in vivo. The unique PK performance of SM-102 makes it an indispensable component in drug development and commercialization.
Five Pharmacokinetic Advantages of SM-102 for R&D
- Ionizable Properties with Neutral Surface Charge – Extended Circulation
- Protonates under acidic conditions to efficiently bind mRNA.
- Becomes nearly neutral at physiological pH, reducing nonspecific protein binding and clearance.
- Compared with traditional cationic lipids, SM-102 significantly reduces nonspecific accumulation in the liver and spleen.
- Rapid Distribution with Controlled Clearance – Precise PK Profile
- After intramuscular injection, SM-102 LNPs rapidly enter the lymphatic system, promoting uptake by antigen-presenting cells.
- Demonstrates fast local distribution and controlled systemic clearance.
- In vivo, SM-102 LNPs achieve peak expression within 24–72 hours and are fully metabolized within 1–2 weeks, reducing long-term exposure risks.
- Predictable Metabolism with Low Toxicity – Regulatory Compliance
- Contains hydrolyzable ester bonds, degraded by esterases into fatty acids and neutral metabolites.
- Meets FDA safety expectations for biodegradable ionizable lipids.
- PK modeling confirms predictable metabolic pathways, supporting PBPK (Physiologically Based Pharmacokinetics) modeling for IND submissions.
- Optimized Tissue Distribution – Efficacy with Safety
- Compared to ALC-0315 and similar lipids, SM-102 shows balanced distribution:
- Lower hepatic accumulation, reducing potential hepatotoxicity.
- Longer retention in muscle and lymph nodes, enhancing vaccine efficacy.
- This distribution pattern makes SM-102 uniquely advantageous for vaccines and localized therapies.
- Tunable Pharmacokinetics – Flexible Formulation Control
- Adjusting SM-102 ratios in LNP formulations alters particle size, surface charge, and release rate.
- In DoE (Design of Experiments) studies, SM-102 concentration directly influences half-life and biodistribution.
Pharmacokinetics Services Powered by SM-102 at Taskcm
- Standardized PK Evaluation Platform
- Plasma concentration–time profiling
- Tissue distribution (liver, spleen, lymph nodes, muscle, etc.)
- Clearance pathway analysis (urine/feces metabolite detection)
- These standardized workflows shorten timelines from formulation optimization to IND submission.
- GLP/GMP-Compliant Systems
- SM-102 is validated in approved products (e.g., mRNA-1273).
- Taskcm leverages existing data packages and GLP methods to accelerate PK study completion and regulatory filings (IND/BLA).
- PBPK Modeling for Clinical Translation
- Supports IM, IV, and SC route simulations.
- Reduces preclinical study cycles and animal usage, lowering costs.
- Customized Formulation-PK Correlation Studies
- Optimization of formulation variables (SM-102 ratio, PEG-lipid type, particle size).
- High-throughput LNP preparation combined with PK screening to identify the optimal PK window.
- Accelerator Role for New Drug Development
- With proven clinical success, SM-102 is packaged as a standard ionizable lipid in LNP platforms.
- Allows R&D teams to bypass lipid discovery, focusing directly on validating their mRNA sequences and indications.
- This dramatically reduces technical and regulatory risks.
Conclusion
As a clinically validated ionizable lipid, SM-102 demonstrates extended circulation, balanced tissue distribution, predictable metabolism, and low toxicity in pharmacokinetic studies. These features enhance in vivo performance of mRNA drugs and create a strong foundation for commercial services.
With standardized PK platforms, GLP/GMP compliance, PBPK modeling, and customized formulation-PK optimization, SM-102 has become the preferred lipid for PK research and industrialization in nucleic acid therapeutics.
Looking ahead, as mRNA drugs expand beyond vaccines into cancer, rare diseases, and protein replacement therapies, SM-102-based PK optimization and services will play an increasingly critical role in accelerating development, reducing risks, and enabling clinical success.