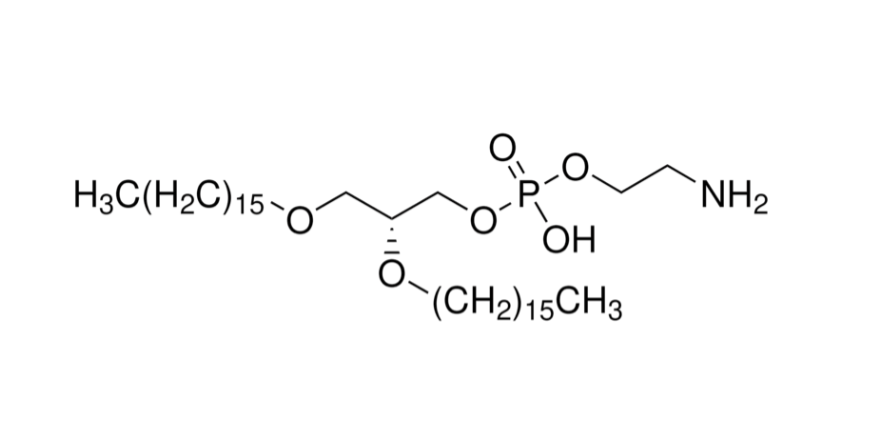
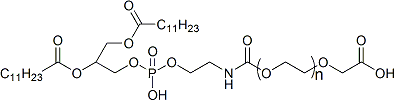
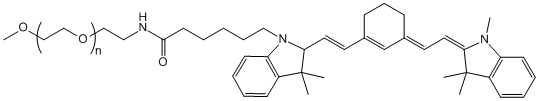
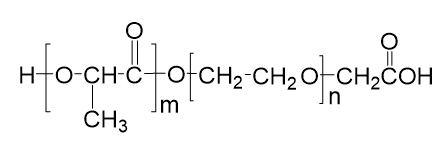
With the growing demand for precision treatment of ophthalmic diseases, Polysaccharide Microneedle Systems (PSMNs) have emerged as a new class of minimally invasive drug delivery technologies, effectively overcoming the barriers of traditional ophthalmic formulations, including limited corneal permeability and low bioavailability.
Leveraging its strong expertise in polymer material development and lipid/polysaccharide delivery systems, Taskcm has successfully developed a series of polysaccharide microneedle systems, including chitosan microneedles, hyaluronic acid microneedles, and alginate microneedles.
The company possesses kilogram-scale manufacturing capabilities and a comprehensive commercialization support system, providing robust technical and regulatory solutions for research institutions and pharmaceutical enterprises in clinical translation.
I. Polysaccharide-Based Microneedle Platform — Safe, Biocompatible, and Biodegradable
Taskcm’s polysaccharide microneedle systems (PSMNs) use natural polysaccharides as the structural matrix, offering excellent biocompatibility and tissue biodegradability, suitable for corneal, transdermal, and mucosal drug delivery.
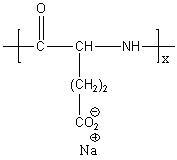
- Chitosan Microneedles: Utilize the cationic and film-forming properties of chitosan to achieve strong adhesion and sustained release on the corneal epithelium.
- Hyaluronic Acid (HA) Microneedles: Leverage the superior hydration and biocompatibility of HA to promote corneal epithelial repair, suitable for conditions such as dry eye and keratitis.
- Alginate Microneedles: Employ ionic crosslinking to form a 3D network, enabling pH- or ion-responsive drug release—particularly advantageous for the delivery of antimicrobial peptides or nucleic acid drugs.
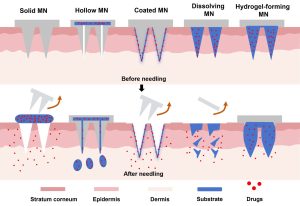
Each microneedle formulation is optimized by Taskcm’s R&D team through precise control of solution concentration, crosslinking ratio, drying methods, and needle tip morphology, ensuring structural integrity and appropriate mechanical strength. Once applied, the microneedles dissolve completely within minutes after penetrating the corneal surface, achieving painless and efficient drug delivery.
II. Kilogram-Scale Manufacturing — Bridging the Gap Between Laboratory and Industry
As polysaccharide-based materials transition from laboratory research to clinical applications, Taskcm has established a robust pilot-scale and scale-up platform, achieving kilogram-level production of various polysaccharide microneedles.
- Chitosan Microneedles: Annual output of 5–10 kg, customizable in molecular weight and deacetylation degree.
- Hyaluronic Acid Microneedles: Manufactured using high-purity, fermentation-grade HA; supports low- and high-molecular-weight hybrid formulations.
- Alginate Microneedles: Enables batch production via calcium ion crosslinking with high product uniformity and process reproducibility meeting international standards.
All materials comply with ISO 10993 biocompatibility standards and are accompanied by complete documentation, including Certificates of Analysis (COA), residual solvent testing, thermal stability, and morphological characterization reports, ensuring quality and traceability throughout research and regulatory submission stages.
III. Comprehensive CDMO Services — Accelerating Industrialization of Polysaccharide Microneedle Systems
As a leading developer of pharmaceutical lipids and polysaccharide-based delivery systems in China, Taskcm provides end-to-end CDMO (Contract Development and Manufacturing Organization) services for polysaccharide microneedle systems—from formulation development to regulatory submission—covering R&D, scale-up, technology transfer, and quality assurance.
1. Formulation and Process Development
- Optimization of microneedle mechanical strength, dissolution rate, and drug loading based on polysaccharide physicochemical properties.
- Provision of multiple fabrication routes including molding, centrifugal casting, and 3D printing, with process parameter validation.
- Development of responsive and controlled-release systems through polymer crosslinking or blending technologies.
2. Quality Testing and Structural Characterization
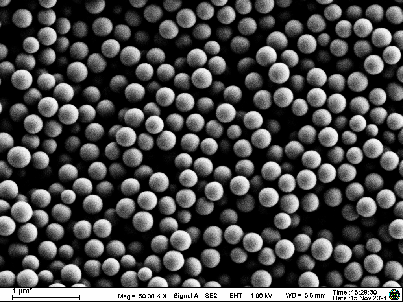
- Comprehensive analysis via SEM, FTIR, XRD, DSC, TGA, HPLC, and GPC, evaluating microneedle morphology, composition, and stability.
- In-depth studies on encapsulation efficiency, drug release kinetics, and biodegradation behavior to ensure reproducible product performance.
3. Pilot Scale-Up and Technology Transfer
- Equipped with GMP-compliant pilot production facilities, independent clean zones, and temperature-controlled drying systems.
- Provides batch consistency validation and critical process parameter (CPP) research.
- Supports industrial scale-up, process optimization, and equipment selection consulting.
4. Regulatory and Compliance Support
- Offers CMC documentation preparation, quality research data compilation, and registration consulting services.
- Experienced in both drug registration and medical device filing, helping clients accelerate approval timelines and reduce commercialization risk.
IV. Innovative Applications — Advancing Microneedle Therapy for Corneal and Dermatological Diseases
The polysaccharide microneedle systems developed by Taskcm have demonstrated significant therapeutic potential in keratitis, dry eye syndrome, and post-corneal transplantation repair.
The company’s PSMN platform can be customized for various target sites with needle lengths ranging from 200 to 600 μm and tailored drug loading strategies to achieve:
- Enhanced local drug penetration and controlled release;
- Polysaccharide–drug synergy for tissue regeneration;
- Long-term immunomodulatory and anti-inflammatory effects.
Beyond ophthalmic applications, Taskcm is extending PSMN technology to dermal aesthetics, vaccine delivery, gene therapy, and localized anticancer drug administration, expanding its potential across biomedical fields.
V. Conclusion — From Laboratory Innovation to Industrial Realization
The commercialization of polysaccharide microneedle systems demands both material innovation and engineering scalability.
With its kilogram-scale production capacity, comprehensive CDMO services, and regulatory expertise, Taskcm stands as one of the few technology-driven enterprises in China capable of delivering end-to-end solutions—from polysaccharide raw materials and microneedle fabrication to pilot scale-up and regulatory submission.
Looking ahead, Taskcm will continue to advance the application and clinical translation of polysaccharide microneedle systems in ophthalmology, dermatology, and vaccine delivery, empowering partners to bridge the gap between laboratory breakthroughs and commercial success, and together pioneering a new frontier in polysaccharide microneedle technology.